Egg quality
The quality of the egg (aka oocyte) is a decisive factor for the success of natural as well as assisted reproduction. Neither the level of reproductive hormones in the blood nor the follicular size can be used to predict oocyte quality. The current benchmark of oocyte maturity is not reflecting its quality.
Benchmarks of egg quality
In the vast majority of cases, oocyte quality is the most important variable determining the success of reproduction. Yet, there is no single clinically useful marker that would predict it. Neither the level of reproductive hormones, the size of the follicles, nor even a visual observation of the oocyte after it is retrieved during IVF, can provide guidance to its developmental potential. Therefore today, oocyte quality can only be defined as a “probability of an oocyte to become a child, once fertilized”.
Oocyte chromosomal maturity
Being unable to evaluate oocyte’s quality prospectively, we can only say whether it is mature, meaning that it has completed the first meiotic division and is at the expected stage of “chromosomal configuration”. This stage can be easily discerned by an observation of the so-called first polar body. At the IVF retrieval, once an embryologist finds that an oocyte retrieved from the follicle has a polar body she will record it as “mature”. The physician considers ovarian stimulation successful if most of the harvested eggs are mature and thus oocyte chromosomal maturity became a synonym of oocyte quality. Yet, we knew for a long time, that the ability of the egg to extrude the polar body is one of the earliest developmental competency benchmarks. This benchmark is reached before the ability to develop after fertilization is attained. Thus the oocyte’s “maturity”, as it is defined today, is not the same as seeing the red color of the tomato and deciding it is ripe. It is more like trying to choose a ripe tomato by looking only at size and shape.

In order to fully appreciate the value of an oocyte’s ability to extrude the first polar body as a competency benchmark, you may think about a newborn developing a sucking reflex. It appears as early as 32 weeks, in the otherwise still immature fetus. Now, imagine that a sucking reflex would be the only benchmark of a fetus’s developmental competency.

You may wonder how this benchmark can be wrong if IVF is so successful. Well, it depends on how you define success. On average only about 30-40% of retrieved oocytes develop to the blastocyst and only about half of them are chromosomally normal. If a female has a lot of oocytes, this is acceptable, but what if she does not?
Follicle size
The reproductive endocrinologist will monitor the size of the follicles during ovarian stimulation and when the largest follicle reaches about 22-25 mm in diameter, she will trigger the ovulation so that the oocytes can be retrieved. This follicular size was chosen because it is close to the follicle’s size just before ovulation in the natural cycle with an underlying assumption that it signifies the oocyte’s competency. However, the size of the follicle and the pace of its expansion is determined by the amount of circulating FSH and the activity of the mural granulosa. It is not influenced by an oocyte and therefore can not tell us anything about an oocyte. That is why, for example, the largest follicle has the same chance to contain the best egg as a smaller follicle.
The misconception that follicular size is a reflection of the oocyte’s properties can be traced to the early stages when the oocyte does indeed controls the follicle (by secreting several specific growth factors). But once the cavity is formed and expanded, the gradient of oocyte-derived growth factors becomes too diluted to reach distal granulose cells. This allows those granulosa cells to escape the oocyte’s control, differentiate into mural granulosa, and begin expressing receptors to FSH. Once this happens, the corona-cumulus complex will remain under the control of the oocyte-produced growth factors. However, the enlargement of the follicle, the result of mural granulosa proliferation and fluid accumulation, will be controlled initially by FSH and later by both FSH and LH. Full recognition of this dual control is very important, because it helps to appreciate that the processes responsible for the egg acquiring competency (nursing with corona-cumulus), and the process responsible for the ultimate egg release from the follicle (ovulation), are driven by completely different, independent, and uncoordinated mechanisms.
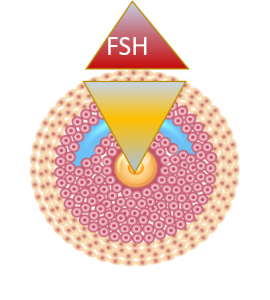
Before the large cavity is formed, egg controls the entire follicle
While the follicle is small, the gradient of growth factors (produced by the egg) prevents the induction of FSH receptors in granulosa. In yellow: a gradient of oocyte produced growth factors: CDF-9, BMP-15, SMAD
Reproduced from Dozortsev and Diamond, Fert Stert 2020, open access publication
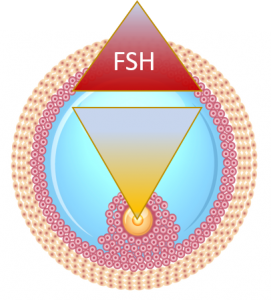
At the recruitment into ovulatory cycle an egg is losing control over the follicle
As an egg is losing control over the follicle to FSH, its time for maturation is at the mercy of the follicle (or an REI). In yellow: a gradient of oocyte produced growth factors: CDF-9, BMP-15, SMAD
Reproduced from Dozortsev and Diamond, Fert Stert 2020, open access publication

This is why just like an obstetrician cannot use the size of the belly to predict a fetus’ maturity, it is not possible to use the size of the follicle to predict an egg’s competence.
Furthermore, during controlled ovarian stimulation, a woman receives additional amounts of FSH, which makes the follicle “grow” about 1.2 times faster than during the natural cycle (in many cases the pace is even higher). At the same time, the pace of the oocyte’s acquisition of its developmental competence is unaffected. This creates a potential for asynchrony between the follicular growth and oocyte acquisition of developmental competence – “term maturation”.
Estradiol
During the follicular phase, estradiol plays multiple roles, some of which we may still not know.
- Suppresses FSH
- Induces progesterone receptors in the hypothalamus
- Induces proliferation of the endometrium, preparing it for implantation
- Enforces LH accumulation in the pituitary, by preventing its release
During controlled ovarian stimulation, estradiol is also used as an indirect measure of an egg’s competence. It is usually assumed that a certain level of E2 per follicle (about 200 pg/ml) predicts an egg’s competence. However, the level of E2 is just another way to measure the follicle, because its main source is mural granulosa, which has everything to do with the follicle and nothing to do with the oocyte.
Follicular stimulating hormone (FSH)
The level of FSH is a mirror reflection of the level of estradiol and therefore cannot predict oocyte quality
Anti-mullerian hormone (AMH)
AMH is produced mainly by mural granulosa of antral follicles and correlates very well with the number of antral follicles and number of oocytes retrieved during ovarian stimulation, but its level is not predictive of oocyte quality.
To sum it up, neither E2, FSH, or AMH nor the size of the follicle cannot be used to predict an oocyte’s competence. Figuratively speaking, they represent different parameters of the box, not its content.